Metallic iron for environmental remediation: A textbook message
The german word for science is “Wissenschaft” and can be word-wisely translated as „knowledge creation“. Knowledge is created by observing and understanding the nature. Once a natural process is understood, the conditions for its reproduction can be artificially created for further experimentation, the final aim being to obtain some benefits for the human beings and the human culture. The art of exploiting the knowledge about natural laws and processes is called technology. In other words, what is generally considered as invention is nothing else as a purposefully mimicking of the nature. The launching of a technology and the subsequent progress in it cannot take place before a theory of the underlying processes is established. The Fe0 iron remediation technology was invented from the observation that loss of chlorinated solvents (RCl) occurred in Fe0/H2O systems. The RCl loss was analytically resolved to be a reductive transformation and the reaction products were characterized. However, Fe0 was wrongly considered to be the reducing agent, as well as the contaminant reduction was considered to be the cathodic reaction taking place simultaneously to Fe0 oxidative dissolution. This confusion has characterized the first two decades of technology development. Achieved results are largely published in highly selective journals, mostly providing a stringent peer review with very low acceptance rate.

Tab. 1. Likely dominant reaction implying contaminant removal in Fe0/H2O systems and the site of their occurrence. * are non stoichiometric.
Controversy is a part of science but is rarely presented in textbooks. Textbooks mostly present final results in form of established hypotheses and models. This evidence suggests that any scientist starting investigations in a field must be familiar with the corresponding textbook knowledge. It appears that the controversy in the Fe0 research arose from the fact that the electrochemical nature of aqueous iron corrosion (that has been established since the 1930s) was not properly considered by the pioneers of the Fe0 technology and the large majority of active researchers.
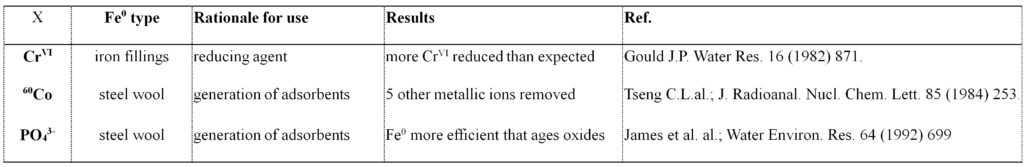
Tab. 2. Overview on the content of three publications on using Fe0 in environment science before 1994. Ref. (Gould J.P. Water Res. 16 (1982) 871) found out that for 1.33 mol of Fe0 dissolved, 1.00 mol of CrVI was reduced. The 1:1 reaction stoichiometry corresponds to the Fe0/FeIII oxidation which is not possible under environmental conditions. Fe0 was purposefully used as generator of iron oxides as early as 1984 (Tseng C.L.al.; J. Radioanal. Nucl. Chem. Lett. 85 (1984) 253).
To stop the propagation of the view that Fe0 is an environmental reducing agent, one has to recall the difference between a chemical and an electrochemical reaction. In a chemical redox reaction, electrons are directly transferred from one species (e.g. Fe2+ or H2) to another (e.g. RCl). Both species are spatially close to each other and the distance between them is some few Å (< 5 Å). In an electrochemical reaction electrons are transferred from one species to another indirectly through an electrode (e.g. Fe0) placed in an electrolyte solution (contaminated water). The electrode is made up of cathodic and anodic sites. Fe0 is oxidized at the anode and water (H2O) is reduced at the cathode (Tab. 1). The distance between anode and cathode depends on the size of the Fe0 particles used (up to 2000 Å). Electrochemical contaminant reduction in Fe0/H2O systems is quantitative if and only if the conduction of electrons left behind by Fe0 oxidative dissolution is assured. The prerequisite therefore is twofold: (i) the Fe0 surface is accessible or (ii) the oxide scale is electronic conductive. However, the oxide scale is a multi-layered physical barrier and non conductive as a rule. Thus, contaminant reduction in Fe0/H2O systems can only result from a chemical mechanism taking place within the oxide scale. The well-documented success of Fe0 filtration systems is attributed to the continuous in-situ generation of iron corrosion products of which some are reducing agents. This knowledge is anterior to the discovery of environmental Fe0 filters and has been used in environmental science in the early 1980s (Tab. 2).
Chicgoua Noubactep
Department of Applied Geology, Universität Göttingen, Germany
Publication
Research on metallic iron for environmental remediation: Stopping growing sloppy science.
Noubactep C
Chemosphere. 2016 Jun












Leave a Reply
You must be logged in to post a comment.