Should we protect the brain barriers to prevent Alzheimer’s disease?
When discovered in 1906, Alzheimer’s disease was described as a peculiar disease and was rarely observed in the population. Today it is one of the top five causes of the death in elderly worldwide. People with Alzheimer’s have a progressive memory loss and their cognitive abilities deteriorate with time. Unfortunately, due to the unknown exact mechanisms underlying this disease, the effective cure has not been found yet. A known negative modulator in Alzheimer’s disease is the beta amyloid 1-42 (Aβ1-42) peptide, which is formed after invalid amyloid precursor protein processing. This toxic amyloid species is able to act on its own in the brain, as well as to aggregate together with other types of amyloid oligomers and form amyloid plaques, the latter being one of the hallmarks of the disease.

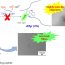
Fig. 1. The choroid plexus is a structure that hangs in the cerebrospinal fluid (CSF) filled ventricles and contains the blood-cerebrospinal fluid barrier, which forms a unique interface between the body (blood) and the brain (CSF).
In healthy conditions, the brain is protected from fluctuations in the blood via the presence of tight barriers. The most studied central nervous system barrier is called the blood-brain barrier (BBB). Another, often neglected and consequently understudied barrier is called the blood-cerebrospinal fluid barrier (BCSFB) and this barrier is located in a small structure in that hangs in the ventricles, called the choroid plexus (Figure 1). Malfunctioning of brain barriers will eventually disrupt brain homeostasis and should be avoided.
Here, we made use of a newly established mouse model of Alzheimer’s disease, which is based on direct injection of Aβ1-42 oligomers into the cerebrospinal fluid of the brain ventricles of mice. Subsequently, we analysed the effect of these toxic Aβ1-42 oligomers on the BBB and BCSFB. Although the effect on the BBB was moderate, we observed severe loss of BCSFB functionality shortly after Aβ1-42 oligomer injection. We could also show that a specific family of endopeptidases, called matrix metalloproteinases (MMPs) are key players in this Aβ1-42 oligomer induced loss of barrier function. Indeed, their activity reduced the tight contacts between the choroid plexus epithelial cells, which are the building blocks of the BCSFB. Consequently, these cells lose their distinctive cuboidal shape and barrier making properties. The most prominent contributor was shown to be one specific member of the MMP family, namely MMP-3, which was already shown to play a role in Alzheimer’s disease and has been proposed as a biomarker. Indeed, both MMP inhibition and MMP-3 deficiency protected mice from Aβ1-42 oligomer induced loss of barrier function.
In conclusion, we have shown for the first time direct toxic effects of Aβ1-42 oligomers on the blood-cerebrospinal fluid barrier which negatively affects brain homeostasis. Interestingly, this can be prevented by blocking the detrimental, Aβ1-42 oligomer induced MMP activity.
Vandenbroucke Roosmarijn E.
Inflammation Research Center (IRC)
Ghent University – Flemisch Institute of Biotechnology (VIB)
Belgium
Publication
Amyloid β Oligomers Disrupt Blood-CSF Barrier Integrity by Activating Matrix Metalloproteinases.
Brkic M, Balusu S, Van Wonterghem E, Gorlé N, Benilova I, Kremer A, Van Hove I, Moons L, De Strooper B, Kanazir S, Libert C, Vandenbroucke RE
J Neurosci. 2015 Sep 16












Leave a Reply
You must be logged in to post a comment.