Unusual behavior of benzoic acid at low temperature
Molecules of benzoic acid form dimers in the crystal, which is doubly hydrogen bonded through the carboxylic COOH groups (Fig. 1.). Since the left and right site of the dimer are fully equivalent, the protons “do not know where they should belong”. Therefore, concerted double proton jumps within the hydrogen bonds from one oxygen atom to another can occur without changing of the total energy of the dimers. This leads to two tautomers, left (L) and right (R) (Fig. 1.), which are also equivalent.
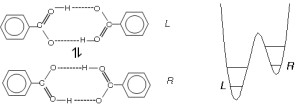
Fig. 1. The two tautomeric forms of the benzoic acid molecules labelled L and R, and schematic representation of the asymmetric double minimum potential. The asymmetry arises at low temperature due to the crystal field.
The to-and-fro proton transfer is known as protonic tautomerism. Upon cooling down to low temperature the protonic transfers stop due to a weak energy asymmetry of L and R tautomers in the crystal. Where, then, the protons of the hydrogen bonds should be located at low temperatures? It was to be expected that they would locate at the midpoints between the oxygen atoms causing a creation of symmetric hydrogen bond. The symmetric hydrogen bonds have been attracting attention for a long time, because strongest hydrogen bonding should arise in the case, but there were no reliable reports on the symmetric hydrogen bonds in the literature. However, benzoic acid permits to observe the appearance of a symmetric H-bond, albite in a rather intricate form. At low temperatures (below 60 K), when all equilibrium vibrations are frozen out and oxygen-oxygen distance is the shortest for a given compound, nonequilibrium vibrations arise in the course of the Raman scattering. (The Raman spectroscopy is a physical method, which permits to excite and observe a vibrational spectrum of chemical compounds). The excited vibrations modulate inter-atomic distances in the molecule, including O…O distance, and provoke its further shortening at least for the time of half the period of the molecule vibration. This short-time getting close of the oxygen atoms is however enough to change significantly the potential well of the proton participating in the hydrogen bond. This means that the proton vibration couples strongly to those intramolecular modes which modulate O…O distance. Due to the coupling the spectrum of the combined modes, that is modes, which are the sum of O-H and intramolecular vibrations, appears in the high-wavenumber region at low temperature.

Fig. 2. A comparison of the high-wavenumber (2400-4000 cm-1, top) and intra-molecular (50-1700 cm-1, bottom) vibrations at T = 10 K.
Figure 2 shows the spectra of intra-molecular (50-1700 cm-1, bottom) and high-wavenumber (2400-4000 cm-1, top) vibrations at 10 K. One can see that the weak lines in the high-wavenumber spectrum are located and spaced in the same order as the bands in the spectrum of intra-molecular (50-1700 cm-1) vibrations. In other words, the spectrum of the weak bands in the high-wavenumber region is really second order spectrum, consisting of the intra-molecular vibrations combined with the O-H stretching mode. The O-H stretching itself is not detected, but its peak position should be at ~2650 cm-1. This observation of the second order spectrum of intramolecular vibrations is very unusual and is reported here for first time.
This unusual behavior of high wavenumber spectra is not a unique property of benzoic acid. A similar feature was observed in Raman spectra of orthorhombic modification of paracetamol. One can also expect similar phenomenon in biological systems, e.g. in complementary pair guanine – cytosine.
Publication
Unusual behavior of benzoic acid at low temperature: Raman spectroscopic study.
Kolesov BA.
Spectrochim Acta A Mol Biomol Spectrosc. 2015 May 5












Leave a Reply
You must be logged in to post a comment.