New advances in the classification of diabetes beyond type 1 and 2: monogenic diabetes
The classification of diabetes has become increasingly complex in recent years, particularly in young patients, and now goes beyond the traditional forms of type 1 and type 2 diabetes. Type 1 diabetes, which is linked to the presence of antibodies that destroy the pancreatic beta cells, usually appears in children or adolescents who will then quickly need to take insulin for life. Type 2 diabetes, which is the most common form, usually appears in adults and is associated with obesity, insulin resistance and the changing lifestyle habits over the last few decades in terms of diet and physical activity. Heredity does not contribute much to type 1 diabetes (less than 5-10% of patients with type 1 diabetes have one parent affected) but has a greater role in type 2 diabetes (the majority of diabetic patients have either one or both parents with the condition).

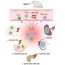
Fig. 1. Distribution of monogenic diabetes
With permission from the author. Can J Diabetes 2016 (Elsevier Copyright 2015)
Monogenic diabetes, which is linked to a single gene passed down from generation to generation in 50% of descendants (dominant autosomal inheritance), is increasingly recognized and would seem to account for 1-2% of all cases of diabetes in young adults. The term MODY (Maturity-Onset Diabetes of the Young) is used to describe at least 13 genes currently identified. The most frequent mutations affect the following genes: HNF1A (over 50% of cases), glucokinase, HNF4A and HNF1B (Fig. 1). These forms have no anti-pancreatic antibodies and retain an ability to secrete insulin, unlike type 1 diabetes. In general, monogenic diabetes affects non-obese people with a normal lipid profile and blood pressure, and who are Caucasians, contrary to cases of type 2 diabetes observed in younger people. Probability calculators designed by the University of Exeter (UK) are available to provide better guidance for clinicians (MODY probability calculator, Diabetes Diagnostics application).
Mutations to the HNF1A and HNF4A genes are responsible for the onset of the disease before the age of 35 (in 80% of cases) in conjunction with a family history of early onset diabetes on the side of the gene-carrying parent. In the presence of a mutation, the secretion of insulin in response to glucose is impaired in the beta cells and this causes progressive hyperglycemia over the course of the patient’s life, which can result in complications if their blood glucose is inadequately controlled. These forms respond much better to low doses of sulfonylurea-like drugs (glyburide, gliclazide, etc.) than they do to drugs such as metformin or insulin, as the first class blocks the ATP-sensitive potassium channels in the cells downstream from the active site of the mutation.
Mutations that inactivate the glucokinase gene (pancreatic glucose regulator) – a fairly common occurrence in the population (prevalence 1/1000 and 1/2000) – cause a mild asymptomatic fasting hyperglycemia (5.5-8.5 mmol/l) from birth, which progresses very little over the course of a person’s lifetime. This form does not seem to result in long-term hyperglycemic complications and does not require treatment with drugs, only the need to follow a healthy lifestyle.
Neonatal diabetes (permanent, temporary, or syndromic) is a rare genetic form of diabetes (prevalence less than 1/100,000) that usually appears in the first six months of life, which is generally a period when type 1 diabetes occurs only very rarely, if at all. Mutations in over 20 genes have been described, some of which respond much better to oral treatments (sulfonylurea) than to insulin.
The identification of these monogenic forms by genetic testing is now increasingly accessible in several laboratories worldwide. Genetic testing costs from a few hundred to several thousand dollars depending on the laboratory and the mutation to be identified. With good selection, genetic testing could be cost-effective given the tremendous impacts over the long term of less expensive and more effective treatments, the cessation of insulin for some cases and the more appropriate monitoring of patients and their families (plasma glucose tests, blood tests, complications, etc.) over the course of a lifetime. Family screening of asymptomatic parents could also be appropriate in certain circumstances.
Carl-Hugo Lachance
Hôpital Saint-François d’Assise
CHU de Québec, Canada
Publication
Practical Aspects of Monogenic Diabetes: A Clinical Point of View.
Lachance CH
Can J Diabetes. 2016 Feb 18











Leave a Reply
You must be logged in to post a comment.