Selective detection of the methyl orange dye among anionic guests
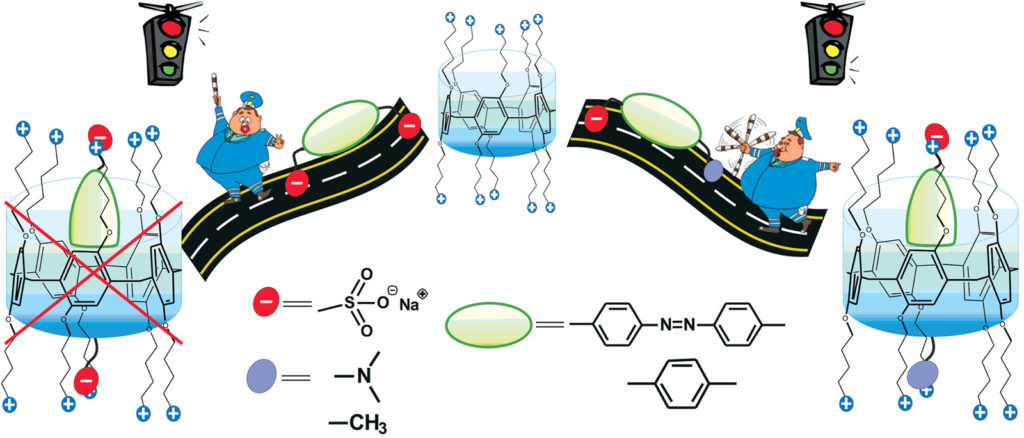
Azo dyes have found various applications in science, medicine and technology. Methyl orange dye is commonly used as pH-indicator that changes its color while protonated is another well-known hydrophobic anion. It is known that water-soluble macrocycles with negatively charged groups bind the protonated methyl orange whereas macrocycles with positively charged groups stabilize non-protonated form of the azo dye. Pillar[n]arenes are new generation of macrocyclic hosts (cage-like organic molecules with accessible internal cavities) for supramolecular chemistry. The macrocyclic cavity of pillar[5]arene has the size comparable to a cavity of cyclodextrines and calixarenes well known as effective complexing reagents for organic and inorganic compounds. Their repeating units are connected by methylene bridges at the para-positions, forming a special rigid pillar-like architecture. The unique structure and easy functionalization of pillararenes allow the synthesis of novel macrocycles able to selectively bind different kinds of guests and provide a useful platform for the construction of various interesting supramolecular systems. The binding of methyl orange with large hydrophobic fragment requires pillar[5]arene with higher hydrophobicity of the cavity. For this reason, we have first proposed to combine these structural fragments in the same molecule in order to increase the depth of the cavity and selectivity of the binding (Fig. 1).
To quantify molecular recognition of the sulfonic acid derivatives by pillar[5]arenes, the stability constants and the stoichiometry of the host/guest complexes formed in the water were estimated by UV-spectroscopy which showed significant changes in the absorbance spectra of the macrocycles after the addition of the guest molecules. The hyperchromic effect was observed at 270-320 nm in case of the guest binding. For methyl orange, changes in the absorbance were monitored at 350-600 nm, while hosts did not absorb light waves (Fig. 2).New adsorption band with maximum near 426 nm was found for the system methyl orange/pillar[5]arene with less than one equivalent of methyl orange added. At its higher concentration, its band was overlapped with adsorption maximum of free guest azo dye (460 nm) in visible spectral region. New band probably corresponds to the complex formation in the system with methyl orange. The association constant Kass value of the complex is 371. The synthesized pillar[5]arenes showed similar binding ability toward sulfonic acid derivatives. In case of the azo dye the appropriate Kass values were 10÷100-fold higher than those calculated for other sulfonic acid derivatives studied. We can propose that the affinity of the guest toward a macrocycle cavity sharply increases with lipophilicity of a guest.
Cationic water-soluble pillar[5]arenes are able to selectively form the 1:1 complexes with hydrophobic anions sulfonic acid derivatives which contain only one sulfonic acid group, but not with the guest molecules containing bulky uncharged or second negatively charged substituents hindering entering the macrocycle cavity. Highly selective binding of the most lipophilic guest, i.e. methyl orange dye, in the form of organic anion salts by positively charged water-soluble pillar[5]arenes is detected.
Luidmila S. Yakimova, Dmitry N. Shurpik, Ivan I. Stoikov
Kazan Federal University, A.M. Butlerov Chemical Institute,
Kazan, Russian Federation
Publication
Highly selective binding of methyl orange dye by cationic water-soluble pillar[5]arenes.
Yakimova LS, Shurpik DN, Gilmanova LH, Makhmutova AR, Rakhimbekova A, Stoikov II
Org Biomol Chem. 2016 May 4














Leave a Reply
You must be logged in to post a comment.