Harmonine – the defense compound of the Asian lady beetle is active against Leishmania major parasites
The Asian lady beetle (Harmonia axyridis) is known to consume large numbers of aphids and mites and has been, therefore, used as biological control of these pests worldwide. However, some populations began to establish locally and H. axyridis became one of the most successful invasive species of our times, threatening and reducing the native lady beetle assemblages wherever it appears.
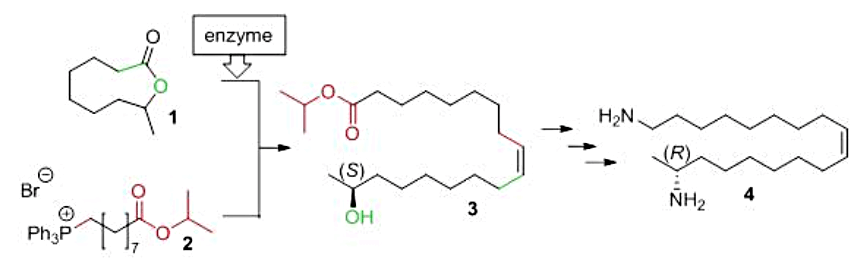
 This invasive success is largely due to the chemical defense the beetle is carrying in its hemolymph (“blood”): the diamino-compound harmonine. Harmonine protects the beetle from predators or infections and displays anti-bacterial activity. These properties arouse a lot of attention lately and more and more possible medical applications of harmonine were studied. Amongst others it displays antimalarial activity and cytotoxicity against certain human tumor cell lines. To prepare larger quantities of harmonine for bioassays an easy, cheap and reliable chemical synthesis of the defense compound was needed. Therefore, we developed a synthesis starting from the lactone (1) and the Wittig-salt (2) shown in Fig. 1. Both compounds can be easily obtained from commercially available products and could be combined in only one step to form the basic skeleton (3) of harmonine. Three additional steps of functional group modification yielded the desired racemic harmonine (4) fast and in high quantities.
This invasive success is largely due to the chemical defense the beetle is carrying in its hemolymph (“blood”): the diamino-compound harmonine. Harmonine protects the beetle from predators or infections and displays anti-bacterial activity. These properties arouse a lot of attention lately and more and more possible medical applications of harmonine were studied. Amongst others it displays antimalarial activity and cytotoxicity against certain human tumor cell lines. To prepare larger quantities of harmonine for bioassays an easy, cheap and reliable chemical synthesis of the defense compound was needed. Therefore, we developed a synthesis starting from the lactone (1) and the Wittig-salt (2) shown in Fig. 1. Both compounds can be easily obtained from commercially available products and could be combined in only one step to form the basic skeleton (3) of harmonine. Three additional steps of functional group modification yielded the desired racemic harmonine (4) fast and in high quantities.
Since the biosynthesis of harmonine is under full stereocontrol (the secondary amino-group has only one orientiation), the beetles produce chiral (17R)-(-)-harmonine (4). To achieve the corret stereochemistry during synthesis, we used horse liver esterase for a highly enantioselective saponification of the lactone (1). Both, the (9S)-(+)-lactone and the resulting (8R)-(-)-hydroxyacid gave access to the enantiomers of (4) in high chemical and optical yield (> 97% ee each). Furthermore, we were able to synthesize potential biosynthetic precursors and derivatives of harmonine along the same concept.
The synthetic harmonine enantiomers were tested in further biological studies to find new, relevant applications. As a target disease we chose the severe tropical disease leishmaniasis. This disease is caused by a parasite, which is transmitted through infected sandflies. The effects on the Leishmania parasite L. major were examined because 1. it is similar to the malaria parasite and the effectiveness of harmonine against malaria was already proven and 2. because medical treatment against leishmaniasis is still lacking. The biological testing showed a strong and comparable activity of both enantiomers, (17S)-(+)- and (17R)-(-)-harmonine against the Leishmania major parasites: observations by transmission light microscopy showed already after 6 hours a different cell structure of the parasite. After 24 hours more than 80% of the parasites were dead.
All in all we did not only synthesize the potent and active molecule harmonine in a short and flexible way, we have also proven that it is active against the parasitic disease leishmaniasis and we managed to synthesize harmonine derivatives, which might also be useful in further drug development.
Nagel N. and Boland W.
Max Planck Institute for Chemical Ecology
Jena, Germany
Publication
Efficient synthesis of (R)-harmonine–the toxic principle of the multicolored Asian lady beetle (Harmonia axyridis).
Nagel NC, Masic A, Schurigt U, Boland W.
Org Biomol Chem. 2015 May 14












Leave a Reply
You must be logged in to post a comment.