Silence the uncontrolled cancer cells’ growth by controlling cellular senescence
When toxic damage caused by environmental or internal stressors accumulates cells can either stop proliferation permanently ( cellular senescence ) or die. Cell death is a common goal of existing cancer therapies, yet cellular senescence is increasingly considered to be an alternative goal. Cells in our body possess a complicated but ingenious senescence-inducing system composed of multiple proteins and pathways. Since excessive proliferative activity caused by oncogenic mutation induces cellular senescence, the response is considered as a protective mechanism against tumorigenesis. Cancer cells, in contrast to normal cells, proliferate infinitely and deprive neighboring cells of nutrients and spaces. While normal cells stop proliferation when they come into physical contact with other cells, cancer cells often keep dividing and therefore form a tumor: a clump of cancer cells. Cancer cells have generally bypassed senescence in order to achieve this infinite growth ability. However, it has been suggested that cancer cells have not completely lost their ability to become senescent, and that the induction of senescence could be used in the treatment of some forms of cancer.

Fig. 1. Flow of screening for senescence inducer compounds (see text). Images of nuclei in the cells treated with compounds were photographed automatically. The compounds altered the size (large) or the texture (spotty) were identified as the “Hit” compounds (Drugs 1~11).
In the present study, we sought to discover small molecule compounds that could induce senescence in normal human cells, which have a complete set of proteins/pathways for senescence induction. To accomplish this, we monitored the appearance (size and texture) of nuclei, because the senescent nuclei are often large and/or spotty (Fig. 1). Cells were treated with hundreds of compounds, and the “facial expressions” of the nuclei were automatically photographed and categorized. In this way, several “hit” compounds, which individually alter the nuclear appearance but do not induce cell death, were harvested. Treatment with the hit compounds actually blocked cell proliferation. Moreover, the treated cells did not “wake up” again or go on to proliferate, even after withdrawing the treatment, indicating that these hit compounds did indeed induced senescence. Thus, the concept of our approach, that reading the facial expressions of the nuclei may lead to the discovery of senescence inducers, was proven.
Among the hits that induce nuclear enlargement, we found ones that gave a very striking nuclear phenotype (Fig. 1, Drugs 1~5). We focused on these hits to examine the detailed molecular mechanisms underlying the senescence induction. Cells treated with these hit compounds had doubled or quadrupled their DNA content, suggesting both nuclear and cell division were impaired. Filming of living nuclei undergoing treatment with the compounds revealed that this was true. As the compounds used were kinase inhibitors and it is known that Aurora kinase B (AURKB) ensures proper cell division and also a strict doubling of the genetic material, we assumed that these hits may inhibit AURKB or its regulator kinases. To our surprise, all the hits turned out to be the inhibitors of the same kinase, AURKB. Consistent with this, genetically engineering a perturbation of AURKB also induced cellular senescence (Fig. 2).
Depletion of the famous p53 tumor suppressor protein from normal cells partially bypasses senescence after AURKB inhibition. Whereas, AURKB inhibition in cancer cell lines, in which p53 is inactivated or deleted, eventually induced senescence. These results indicate that cancer cells hold onto their ability to become senescent after AURKB inhibition and that p53’s contribution to this process is partial. Moreover, we could only induce senescence in dividing cells, not in resting cells, suggesting that AURKB inhibition would only selectively affect actively dividing cancer cells. In summary, our experimental results show that hunting for senescence inducers may be a novel avenue to discover novel anticancer agents. It is known that cellular senescence is associated with secretion of factors, including cytokines and chemokines, which could promote tumorigenesis and therefore should be considered as a side effect of therapy. In the senescent cells induced by AURKB inhibition, however, the secretion of these factors, or at least the ones we tested for (IL-6, IL-8, and MMP3), was not increased. AURKB inhibitors have already been subjected to clinical trials, but it would be good to revisit the reasoning to target this kinase in cancer therapy.
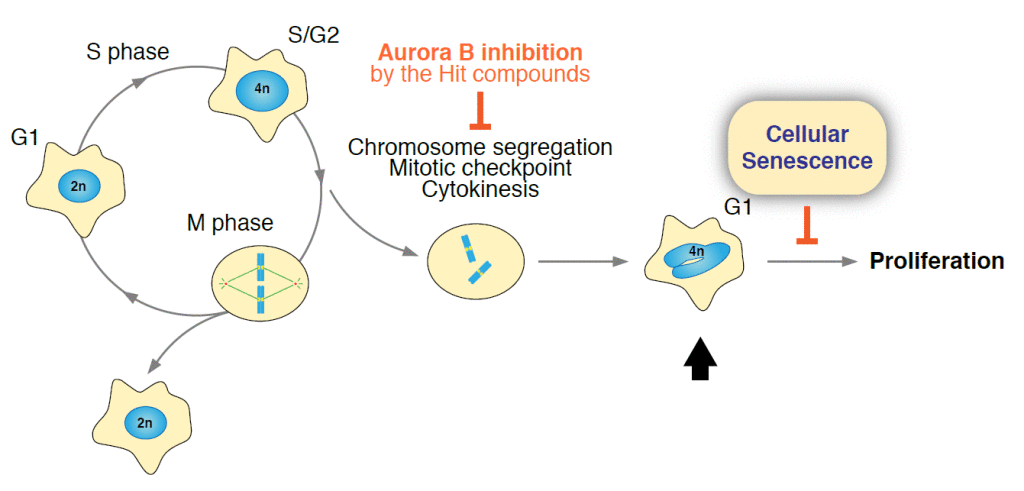
Fig. 2. A subset of the hit compounds (Drugs 1~5) that induce enlargement of nuclei are the inhibitors of Aurora B kinase (AURKB). AURKB inhibition causes failed segregation of doubled chromosomes, cancel of mitotic checkpoint, and cytokinesis defect. This produces the cell with irregular-shaped nucleus containing one excess set of chromosomes (marked by arrow). Proliferation of this abnormal cells is prevented by cellular senescence.
Our approach could lead to the discovery of novel players in cellular senescence. This in turn allows us to understand how senescence is hampered in cancer cells, and to develop strategies for cancer therapy utilizing senescence re-activation. Moreover, the senescence inducer identified by our approach could readily be a promising anticancer agent if it induced senescence exclusively in cancer cells. Importantly, we found that a certain type of cancer cells showed higher sensitivity to the hit compounds than normal cells. Besides developing therapeutic approaches that provoke cell death, the development of pro-senescence (senescence inducing) agents should break alternative ground in the field of anticancer drug discovery.
Publication
Cell-based screen for altered nuclear phenotypes reveals senescence progression in polyploid cells after Aurora kinase B inhibition.
Sadaie M, Dillon C, Narita M, Young AR, Cairney CJ, Godwin LS, Torrance CJ, Bennett DC, Keith WN, Narita M
Mol Biol Cell. 2015 Sep 1













Leave a Reply
You must be logged in to post a comment.